From counting calories to tracing COVID-19, there are hundreds of thousands of health and wellness apps on the market, and the thirst for them is only growing. However, many have access to highly sensitive personal information whilst others may offer advice that is not always supported by scientific evidence. In order to evaluate the quality and reliability of such apps effectively, a new technical specification has just been published.
CEN-ISO/TS 82304-2, Health software — Part 2: Health and wellness apps—Quality and reliability brings together and builds on guidelines and requirements for apps by many local and national health organizations around the world to ensure they are safe, reliable and effective.
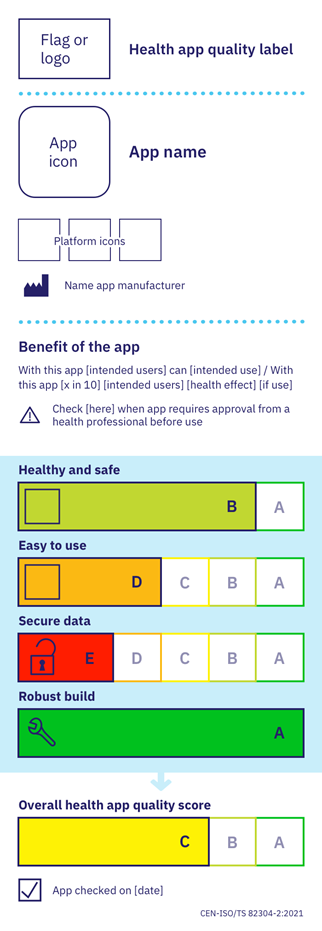
Recommended by the European Union in their European toolkit for COVID 19 tracing apps to “filter out qualitative and trustworthy health apps“, the guidance provides an internationally-agreed set of specifications to assess the apps, with a scoring methodology that gives a ‘traffic light’ themed label. The label enables apps to be easily compared by users and health professionals.
The technical specification will help the industry for health apps realize its potential in managing chronic diseases, tackling unhealthy lifestyles and supporting ageing populations. It also provides a useful tool to promote the use of good quality health apps where healthcare budgets are stretched and where there are disparities in quality and access to health services.
CEN-ISO/TS 82304-2 was developed by ISO technical committee ISO/TC 215, Health informatics, in collaboration with IEC/TC 62, Electrical equipment in medical practice, of the International Electrotechnical Commission (IEC). The project was led by CEN, the European Committee for Standardization.
CEN-ISO/TS 82304-2 can be purchased from the NENshop, your national standards organization or the ISO Store.