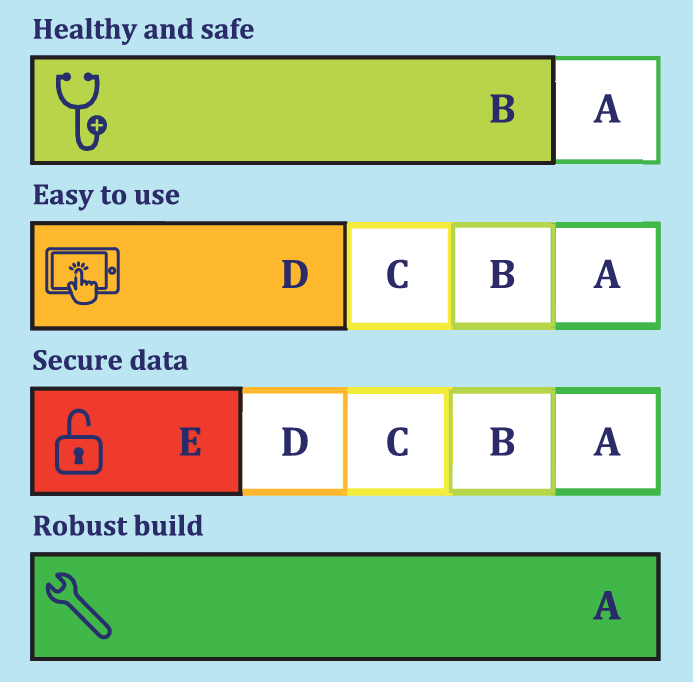
The CEN and ISO committees on Health informatics as well as IEC/TC 62A have approved CEN ISO 82304-2 in ballot. 95% of the participating countries voted positive. The project team has resolved the editorial comments and decided to submit the document to ISO for publication.
The ballot also proposed technical comments. The project team decided not to resolve these comments as this would have resulted in an additional ballot and subsequent delay. The technical comments will be resolved in a future revision of EN ISO 82304-2.
The Horizon Europe solicits a Coordination and Support Action to support the implementation of CEN ISO 82304-2. Contact zw@nen.nl for access to the (draft).