TC 251 active projects
TC 251 closed projects
Dissemination has been an on-going part of the IPS Project from its inception, where the CEN and HL7 teams have presented, written and explained what the IPS is, what it can do, and why it is so important. With the addition of the SNOMED CT IPS Free Set and the IHE IPS Profile, as well as the adoption by ISO of the now EN ISO 27269 International Patient Summary standard, we have created a consolidated IPS website that provides explanations, examples and links to the global standards and specifications that together make up the International Patient Summary.
One particular attempt to capture the story-line of the IPS, and thereby ensure the compelling arguments and rationale are not lost, is the CEN IPS Prezi, a presentation which is intended to complement rather than duplicate the IPS standards. The link to the CEN IPS Prezi can be found here.
xShare: Expanding the European EHRxF to share & effectively use health data in EHDS
xShare envisions that everyone can share their health data in EEHRxF with a click-of-a-button.
The xShare button featured across health portals and patient apps, allows people to exercise their data portability rights under GDPR. Hence, the EEHRxF will enable everyone to safely share their trusted health data with a click of the xShare button and will be the driver for research and innovation in the European Health Data Space (EHDS). The EEHRxF stands for the “European EHR eXchange Format” introduced by EC recommendation in 2019 and further developed by the XeHealth (2020-22) and myHealth@EU.
In digital compass 2030, European Union (EU) empowers businesses and people in a human-centred, sustainable, and prosperous digital future. xShare aims to fulfil this ambition for the health sector. With simple sharing of high-quality trusted health data, participation in the digital health economy becomes effortless contributing to digitally-resilient individuals and healthier societies, where citizen science flourishes and data inform decisions in health systems.
xShare aspires to drive with EEHRxF Europe’s competitive advantage for research & innovation in the EHDS. With trust and flow of high-quality structured coded health data, people will be digitally-competent, data-literate, and thus, better equipped to make decisions in the fight against cross-border health threats. At the confluence of the xShare movement, Europeans will be able capture value from their EHR data in high quality, enhancing the 100% EHR access target of digital compass 2030 and supporting digital health services to move without restriction within the EU. In a simple and straightforward way, individuals will be able share their health data in harmonized structured and coded formats across digital health services and Europe will be influencing global digital health standards.
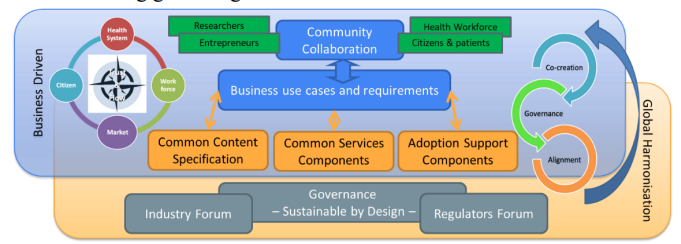
Figure 1: The EEHRxF Standards and Policy Hub engages the digital health community with industry and regulators.
xShare aims to create the EEHRxF standards and policy Hub led by CEN (R. Stegwee) and DIGITALEUROPE (M. Strübin). The Hub engages 6 key Standards Developing Organizations (SDOs) i.e. CEN, HL7 Europe, IHE Europe, SNOMED, CDISC, IEEE, with competence centers and the digital health industry in 13 EU and EFTA member states. This partnership, sustainable-by-design, advances EEHRxF development, maintenance, and adoption for the benefit of European citizens, the resilience of health systems, and the competitiveness of the European industry (see Figure 1).
In the Hub, the xShare community will research, develop and harmonize EEHRxF categories across priority health information domains applied in three traditional verticals in the health sector:
(1) portability of health care for continuity of care (EHDS-1, primary use of data, WP3 led by M. Marques of Smart4Health and A. Berler of Gnomon Informatics), (2) population health & cross-border health threats (EHDS-2, secondary use of data, WP4, led by L. Nicolas of EHTEL and E. Rinaldi of Charite), and (3) clinical research (EHDS-2 secondary use of data, WP5, led by D. Kalra of I~HD and Y. Matsakis of EUCROF). Each of these research and innovation tracks is co-led by leaders long engaged in interoperability actions globally.
Horizontal activities will be dedicated to benchmarking and evaluation of EEHRxF adoption within and without xShare (WP6, led by V Stroetmann of empirica), capacity building emphasizing data and digital literacy, procurement as well as security and privacy linked to EEHRxF in different business use cases (WP7, H Martins of ISCTE and XpanDH, the support action building the EEHRxF ecosystem). Dissemination and Communication, and Stakeholder engagement are led by ECHA, K Mackiewicz) complemented synergies with DigitalEurope Update (empirica), myHealth@EU (HL7/IHE), and HealthData@EU (HealthDataAuthority and Sciensano).
Part of the budget will be dedicated to open calls aiming the further accelerated EEHRxF adoption across Europe, EFTA, and the accession countries. Building on the BlueButton concept, explicitly transferring and harmonizing across verticals in the EHDS, the xShare proposal explores the feasibility of a European Industry label EU-xShare (WP9, DIGITALEUROPE, led by M Strübin).
When the EU-xShare label is featured in patient portals and trusted apps, it will allow individuals to: (a) receive (i.e. produce) their structured and coded health data in EEHRxF and (b) share their health data with applications that will consume trusted high quality health data associated with appropriate provenance information. The xShare Research and Innovation Action is driven by pioneers and enthusiasts of digital health interoperability led by MedCom (DK, administrative, and HL7 Europe Scientific/Technical). They recognize that it is time to establish the EEHRxF Standards and policy Hub as a sustainable-by-design partnership of SDOs and the digital health industry that taps on the potential of the upcoming EHDS regulation bringing digital health transformation to the cultural and operational tipping point.
The xShare consortium brings together 37 key industry associations, government agencies, competence centers, and SMEs to create a sustainable Standards and Policy Hub that champions health standards as infrastructure for research and innovation in Europe. Additional renumerated experts further expand the outreach of the xShare initiative to drive the global health standards harmonization movement.
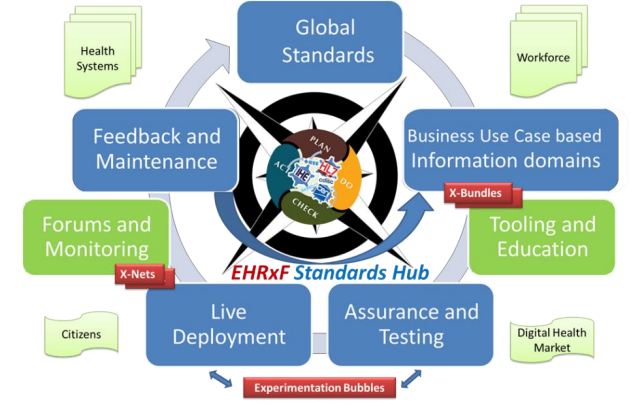
Figure 2: The EEHRxF Standards and Policy Hub builds on XpanDH concepts to incrementally improve interoperability through Plan-Do-Check-Act Cycles research, development and harmonization of EHRxF.
xShare Trust & Flow aims to serve as research and innovation accelerator for the EHDS radically advancing the quality of EHR data available for research and policy oversight.
The ambition of xShare is to serve as an incubator of policy acts, demonstrating the implications of implemented and delegated acts. This is the essence of eStandards as infrastructure for innovation introduced in the eStandards roadmap of 2018, the evolution of which is presented in the figure above, showing the link to the XpanDH EEHRxF ecosystem.
This way, xShare will measurably show the value of EEHRxF harmonized across continuity of care, clinical research, and population health and will:
• Facilitate the movement of digital health services using EEHRxF.
• Explore feasibility and value of EU-xShare industry label for indicating EEHRxF support.
• Build the EEHRxF Standards and Policy HUB sustainable by design.
The aim of the project was to participate in the creation of an International Patient Summary specification, at a global level, and turn this European knowledge and interests into a European Standard, in line with the Guidelines adopted by the European eHealth Network (eHN).
Formal Deliverables have resulted in:
The project team (PT) comprises the following experts:
Context
This proposal addressed the 2015 Rolling Plan on ICT standardization and in particular the work of the Joint Action Supporting the eHN.
This proposal builded upon and formalised the revised Patient Summary (PS) dataset that was initially proposed by the eHN in 2013; its second revision was then officially adopted by the eHN in November 2016.
The Multi-annual Work Plan (2015-2018) of the eHealth Network identified both the work on the cross-border exchange of PS data and the alignment of standardization activities in eHealth as key priority areas. The European eHealth Digital Services Infrastructure (eHDSI) has also been tasked with a deployment project across member states using the eHN PS guidelines as part of their work. With this in mind, the CEN proposal looked to national, regional and international work in PS to produce consistent, implementable Patient Summary standards.
The wider international context, the EU-US collaboration in particular, was taken into account by this proposal. In parallel, HL7 produced its IPS standard and, concurrently, the Joint Initiative Council on Global Health Informatics Standardization (JIC) produced an informative guideline, cataloguing a set of implementable standards for the exchange of a Patient Summary (published in 2017).
For this project, CEN/TC 251 worked together with the Horizon 2020 PHC34 CSA projects, particularly Trillium Bridge and eStandards, and these provided support for the Internationalisation of Patient Summary standards. The Trillium II project, also promoted the IPS work, was instrumental in bringing together two of its participating SDOs, HL7 International and CEN/TC251, to agree a common mission and scope for an International Patient Summary in April 2017.
This very successful collaboration became known as the IPS Project, and the two partners known as CEN IPS and HL7 IPS respectively. A new European eHAction roadmap (2018-2020) seeked to leverage the work of the IPS Project going forward so as to support the eHDSI initiative.
The i2X (“Intelligent Implementations of the EHRxF”) project has a duration of 48 months and it will start on
1st of April 2025
Implementing in-service exemplars through usage of European Electronic Health Record Exchange Format
(EEHRxF) is the way to demonstrate that the industry as well as the healthcare providers can embrace, and
benefit from EEHRxF implementation. The i2X efforts will support industry, directly and indirectly, as it aims
to provide an opportunity for testing the in-service use of the EEHRxF in clinical settings, many of which are
public or strongly oriented for public service.
The i2X project will deploy experimentation within different real-world contexts, emphasizing the
correlation between data processing and patient care, and underlining the necessity to redesign the user
experiences of both “healthcare professionals” and “patients”.The i2X project gathers 42 participants
around Europe, of which 18 are private companies operating in the Digital Health market and will engage
even more within the project ecosystem. In addition, the consortium brings together a broad participation
of SMEs from different geographic areas and large health IT companies in order to facilitate and promote
opportunities for joint ventures and technical knowledge sharing. The i2X demonstrators were largely
suggested and brought in by companies, to build trust and to increase the uptake of EEHRxF.
CEN/TC 251 experts are actively participating in work packages and related tasks.
UNICOM is an Innovative Action project supported by European Commission, focusing on implementing the standards for Identification of Medicinal and pharmaceutical Products (IDMP) as developed, published and maintained by the International Organization for Standardization (ISO). We understand that a full and coordinated set of IDMP-related standards and terminologies will be needed to make it work in real life. Our work will therefore involve further development, testing and implementation to support:
The 4-year project has objectives focusing on a number of areas in support of the implementation of IDMP for the above purposes:
Next to CEN/TC 251, SNOMED International, GS1, IHE, HL7 and others are all part of the project consortium and committed to ensuring their relevant standards can work together in support of project requirements whilst also considering long term sustainability beyond and outside the project.
Visit the website of UNICOM.
You may already have seen mention of a new project on Expanding Digital Health across Europe – the XpanDH project. CEN/TC 251 is proud to be involved in the XpanDH project, which kicked off early in 2023. This project aims to expand the use of the European Electronic Health Record Exchange Format (EEHRxF) for use in Digital Health across Europe and beyond.
The EEHRxF was established following a recommendation from the European Commission in 2019. Drafted during the X-eHealth project, it is expected to form the cornerstone of the European Health Data Space as soon as the proposed regulation has been accepted by the European Parliament and the European Council and formally published. It also is at the heart of the MyHealth@EU infrastructure for cross-border health information exchange.
CEN/TC 251 experts actively participate in the following XpanDH work packages:
Should you have any questions or interest in participating, please don’t hesitate to reach out to CEN/TC 251 Chair, Robert Stegwee. We have set up a Review Group for this project within CEN/TC 251, to share insights, progress, and solicit comments on draft deliverables from the project. Should you wish to become member of this Review Group, please make sure you are a designated representative of your national standards body and inform the TC 251 secretariat of your interest in joining.
Below we include a press release on the first XpanDH consortium meeting in February 2023 in Lisbon. More information can also be found on the recently launched XpanDH website.
In cooperation with the international standardisation experts in ISO/TC 215/JWG7, a specific expert team in CEN/TC 251/WGII is working on delivering CEN/TS 82304-2 ‘Health software – Part 2: Health and wellness apps – Quality and reliability’.
This project has been started at the request and with the support of the European Commission. It is due to be completed end of 2020. It will help to establish a common framework across Europe for the evaluation of these apps, giving users and health professionals confidence that the apps are fit for purpose, and providing app developers easier access to European markets.
Europe is experiencing a fast growing market for Health and Wellness Apps. At the same time, concerns about the quality and reliability of apps have risen. Many Health and Wellness Apps are being published without clarity on the level of quality and reliability.
This specification addresses the specific needs of the developers of Health and Wellness Apps:
The project team includes members from the following CEN member countries: France (AFNOR), United Kingdom (BSI), Germany (DIN), Netherlands (NEN), Sweden (SIS), Finland (SFS) and Italy (UNI). Quoting the project leader Charles McCay: “Initial work for this standard has already been done in the United Kingdom with the publication by BSI of the publicly available specification PAS 277. This project will expand that work to meet the wider European requirements and ensure compatibility with the world health informatics standards from ISO and IEC.”
Those interested in participating on this project should contact their national standards organisation via ISO and/or CEN-CENELEC.
For more information on European standardization activities in relation to Health, please see this CEN website.
This multi-stakeholder project ran between January 1, 2017 and September 2019, in which NEN (representing CEN/TC 251) was one of the 25 partners from Europe and US.
TRILLIUM II was a EU/US Cooperation for Global Interoperability in Digital Health and has advanced an International Patient Summary standard to enable people to access and share their health information for emergency or unplanned care anywhere and as needed starting with immunizations, allergies, medications, clinical problems, past operations and implants.
CEN/TC 251 has lead WP5 ‘EU/US eHealth Interoperability Roadmap, Open innovation and International Patient Summary Standards Governance’ which resulted in two specific deliverables.
Go to the website of Trillium II for more information.
ssembling a shared commitment of 47 health actors, the underlying idea of this project is to develop the basis for a workable, interoperable, secure and cross border Electronic Health Record exchange Format in order to lay the foundation for the advance of eHealth sector while using the 3 pillars put forward by the EC as reference.
Aimed at promoting a faster and sustainable EU digital transformation, this Cooperative and Support Action is made up of 8 Work Package in which 4 exclusively focus on technical-functional activities (WP4 to WP7). From Generic Aspects to System Architecture and Integration, passing by Functional and Technical Specifications, X-eHealth objective is to move towards a uniform interoperable data-sharing format framework. In addition, to enhance EU’s public health state of play, WP1 and WP8 are responsible for implementation studies, practicality and continuity of eHealth interoperability development.
On this basis and building upon the already in place Patient Summary, X-eHealth purpose is to develop the foundations for a common framework for medical imaging, discharge letters, laboratory results and rare diseases to flow both alongside citizens care pathway and across health entities between EU Member States and Neighbour Countries.
Focus on cross-border services, this consortium aims to advance an interoperable Common European Health Data Space for citizens and health providers engagement in accordance with privacy and cybersecurity regulations.
To achieve this end, X-eHealth gathers 36 consortium partners plus 5 collaborative partners and 6 eHealth skilled experts, eager to develop the above mentioned 4 domains, and distinguished by policy and political actors mixed with national competent authorities to indeed concretely plan, implement and maintain national eHealth infrastructures.
This TC is involved in:
More information about this project can be found here.