ISO TS 82304-2 ‘Health solftware – Quality and reliability of health and wellness apps’ is published for comments. Till May 14 it is possible to comment on the method to assess whether health and wellness apps are reliable and safe.
Appstores contain a huge amount of health and wellness apps. Apps to quit smoking, to count steps, or to eat more healthy, to guide lifestyle change. Important to know whether the apps are reliable and safe. ISO developed a method to assess the quality of health and wellness apps. The results are used to calculate a score for a label. The label assists you to compare apps and to select the one that suits your needs.
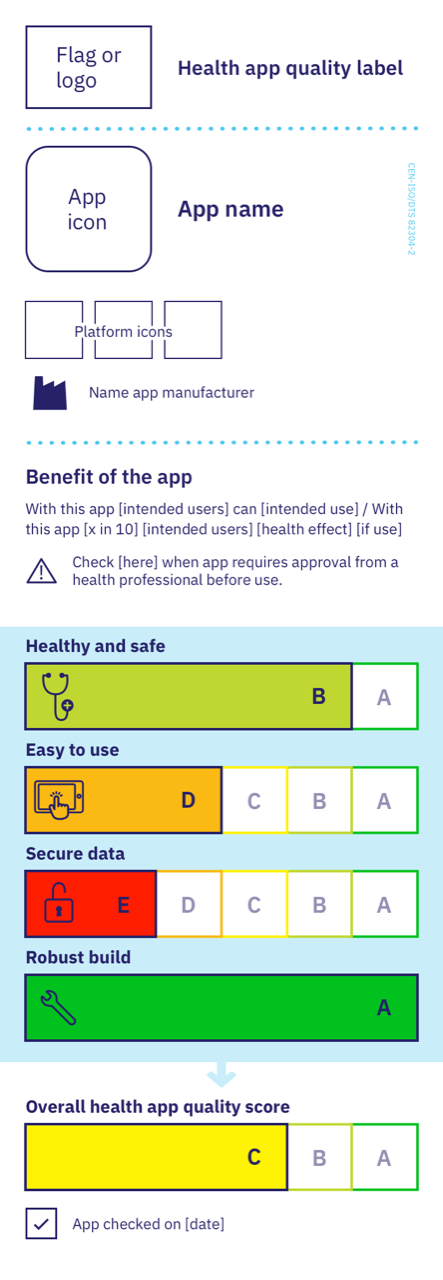
The label
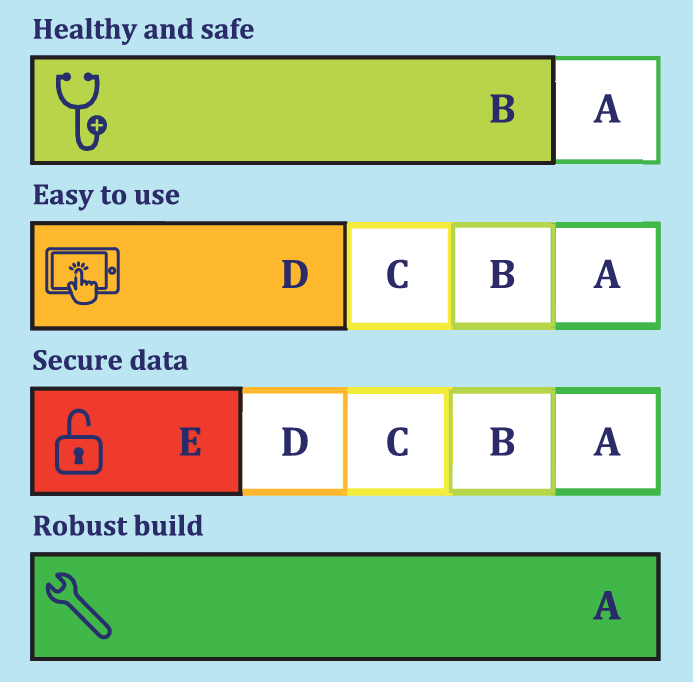
The new draft ISO standard (ISO TS 82304-2) proposes a set of 81 questions for app developers and manufacturers. Based on the answers and evidence provided the health app qualifies for an health app quality label with a score on four topics:
• Healthy and safe;
• Easy to use;
• Secure data;
• Robust build.
The questions and the label with its colour code support health professionals and users of apps to better select apps and app developers to better design apps.
Who
The standardization communities in CEN, CENELEC, ISO and IEC on health and wellness apps have jointly developed the set of questions to assess the apps. CEN/TC 251 had the lead. Additional international experts reviewed the questions and the scoring methodology in a Delphi study.
The European Commission and the European mHealth Network are closely monitoring the developments and are eager to see the results of transparency and reliability of health apps for deployment in health care.
Comment
Till May 14 you can access the draft document and comment on its content. The standardization group will resolve the suggestions and publish EN-ISO TS 82034-2 Software development – quality and reliability criteria for health and wellness apps.
Contact your national standardization organization for access to the document and the commenting procedure.
More information
For more information please check the website or contact Evelyn Noordam or Marlou Bijlsma, e-mail zw@nen.nl.